The humble, or not so humble, Portobello mushroom may one day be the driving force behind electric cars, smartphones and more. This comes after researchers at the University of California’s Riverside Bourns College of engineering created a new, eco-friendly, cost-effective lithium battery using the Portobello mushroom.
According to the research report published in the Nature Scientific Reports journal, researchers found that the highly porous quality of the mushroom creates more space for the transfer of energy. This is a critical component to improving battery performance.
The anode is the part of the battery through which current flows. The new anode, created using the organic material, is set to replace the industry standard of synthetic graphite. The conventional method of preparing graphite anodes involves harmful chemicals and is activated by bases that are not environmentally friendly. This conventional method is also expensive so scientists have worked to cut costs to pocket and to the environment.
Researchers predict a spike in battery use is on the horizon as technology becomes more and more dependent on battery power. This will make the expensive, conventional anode creation process unsustainable in the future, making the move to natural forms all the more important. But how does the product work?
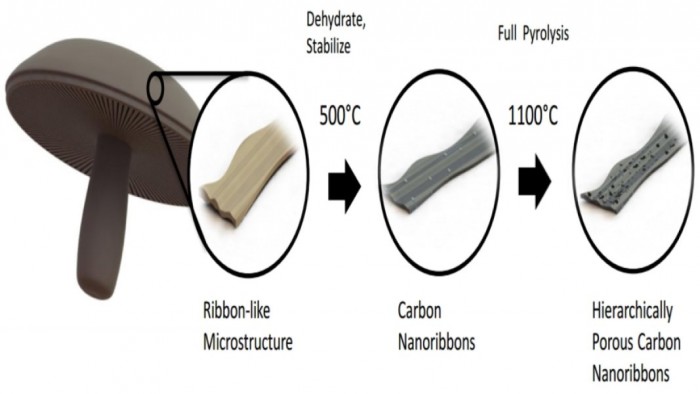
According to the research, Portobello mushrooms are turned into anodes by reducing the fungi tissue into carbon nanoribbons through a process of heating and reheating. The heat removes unneeded compounds while leaving the carbon intact. This carbon is layered with tubes varying in size, all invisible to the naked eye.
Additionally, the high potassium salt concentration in mushrooms allows for increased electrolyte-active material over time by activating more pores, gradually increasing its capacity. This means the normal smartphone battery will get better the longer you use it as opposed to the brutal struggle of a smartphone battery that only works when plugged into an outlet.
"With battery materials like this, future cell phones may see an increase in run time after many uses, rather than a decrease, due to apparent activation of blind pores within the carbon architectures as the cell charges and discharges over time," said Brennan Campbell, a graduate student in the Materials Science and Engineering program at UC Riverside.